About Us
Sunway Medical Centre's Clinical Research Centre (SunMed CRC) was established in August 2009 in response to the demand for evidence-based practice as well as Sunway Medical Centre, Sunway City's desire to actively participate in medical research. Our mission is to advance medical knowledge and patient care through clinical research.
There are 2 main types of clinical research

Clinical Trial
A study that involves interventions like drugs, medical devices and treatments.


Observational Study
A study that involves human participants.
Clinical Trial
A clinical trial is a research study that involves testing the safety and efficacy of medical interventions, such as drugs or devices. In this research study, researchers assign participants to receive one or more interventions to test their effects in people. Because of this, a clinical trial is also referred to as an interventional study. Often, the intervention is investigational, meaning it has not yet been approved for doctors to prescribe to people.
Why Participate in a Clinical Trial?
Participating in a clinical trial offers several benefits:

Access to new treatments
A clinical trial provides access to new cancer treatments that are not yet available to the public.
Contribution to medical research
By participating in a clinical trial, you help researchers learn more about cancer and help future cancer patients.
Close monitoring
If you participate in a clinical trial, you will be closely monitored by a team of doctors and nurses.

Types of Clinical Trials
There are several types of clinical trials, including:

Treatment Trial
A treatment trial tests new treatments like a new cancer drug, a new approach to surgery or radiation therapy, a new combination of treatments, or new methods such as gene therapy.

Prevention Trial
This type of trial seeks to identify better ways to prevent disease in individuals who have never had the disease or prevent the recurrence of the disease.

Screening Trial
A screening trial assesses the best way to find cancer, especially in its early stages.

Quality of Life Trial (or Supportive Care Trial)
Such a trial explores ways to improve the comfort and quality of life for cancer patients.
THE PHASES OF A CLINICAL TRIAL
A clinical trial is conducted in phases, with each designed to answer different research questions:
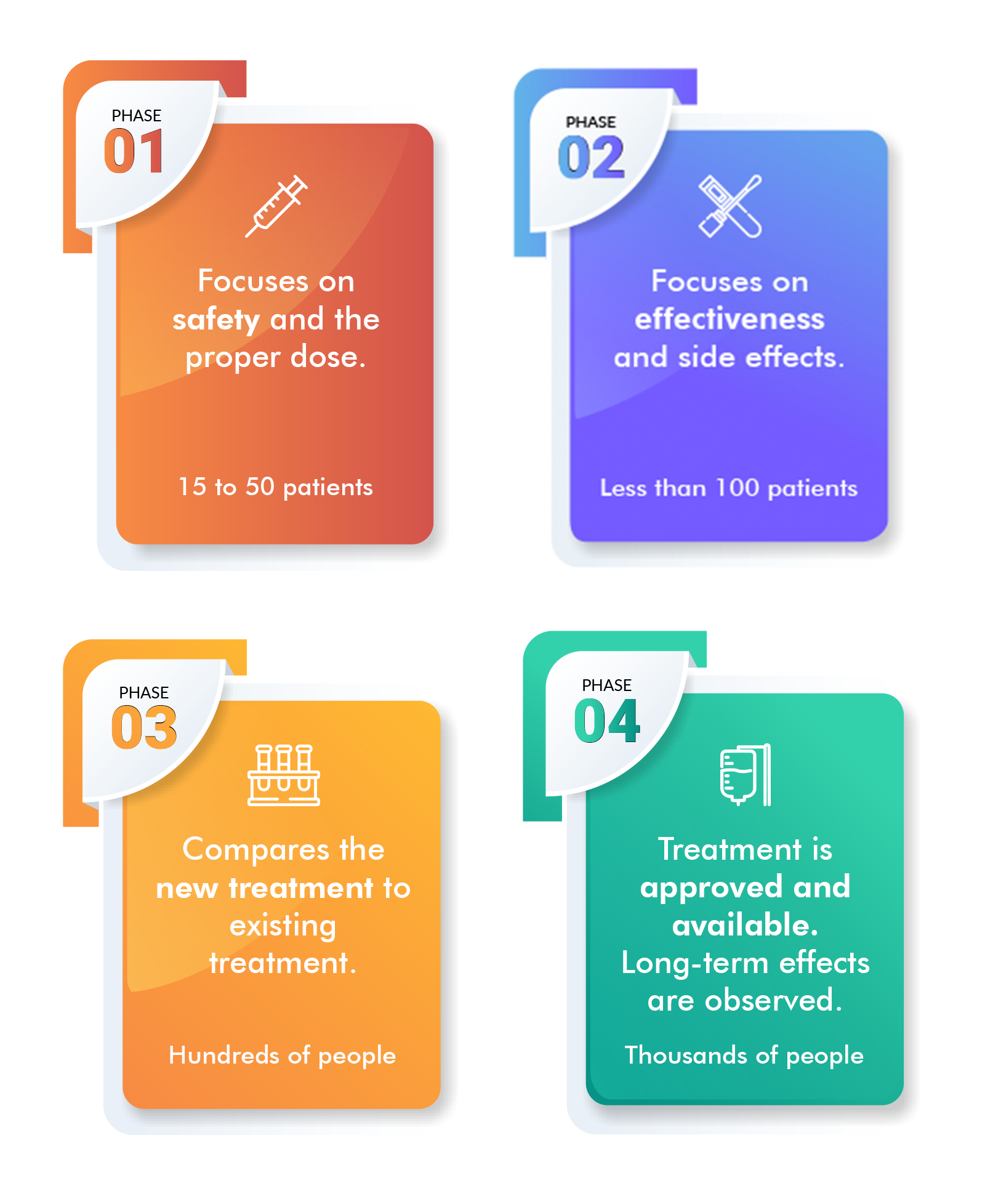
Phase I trial
During a Phase 1 clinical trial, the research team determines if a new treatment is safe, identifies its side effects, assesses if it can be tolerated by people, and establishes the maximum dose that can be safely administered. This phase is conducted with a small group of participants, typically between 15 to 30 individuals. An additional objective of this trial is to confirm that the treatment has an impact on the cancer.
Phase II trial
In a Phase 2 clinical trial, the participant pool is expanded to include more individuals, typically between 50 to 100. The aim of this phase is to evaluate the effectiveness of the new treatment against cancer, such as its ability to shrink tumors or slow their growth. Researchers are interested in understanding how the new treatment interacts with the body and combats cancer. Safety continues to be a priority in this phase, with a focus on monitoring short-term side effects.
Phase III trial
During a Phase 3 clinical trial, the new treatment is compared with the existing standard treatment to determine which one is more effective. The side effects of both treatments are also compared. To ensure that any observed differences are due to the treatments themselves and not due to variations in participants, individuals are randomly assigned to receive one of the treatments. A Phase 3 trial involves a large number of participants, ranging from 100 to several thousand, to validate the results. The outcomes of Phase 1 to 3 trials are crucial in making decisions regarding the approval of new treatments or the use of existing treatments for new conditions. Regulatory agencies, such as the US Food and Drug Administration (FDA), rely on these results for their decision-making process.
Phase IV trial
A Phase 4 clinical trial is conducted to evaluate the long-term safety and effectiveness of a new treatment after it has been approved by the FDA and made available to the public. The safety and effectiveness of the treatment are monitored in large and diverse populations. As the drug or device is used by more people over an extended period of time, additional information is collected.
How does a clinical trial work?
A clinical trial occurs in four phases, and each phase has a different purpose.





The following terms are often used in describing clinical trials

Randomisation
The process in which treatments are assigned to participants in a clinical trial by chance rather than choice. Neither the researcher nor the participant selects which treatment a given participant will receive.

Placebo-Controlled Clinical Trial
A clinical trial in which the treatment of interest is compared to a control group that receives a placebo (a treatment that resembles the investigational treatment but does not have any active ingredients).

Open-Label Clinical Trial
A clinical trial in which both the participants and the researchers know which treatment is being administered to the participants.

Single-Blind Clinical Trial
A clinical trial in which the clinical trial research team is aware of the treatment a participant is taking, but the study participants do not know which medicine or treatment they are receiving.

Double-Blind Clinical Trial
A clinical trial in which neither the study participants nor the researchers know what treatment the participants are receiving until the study is complete. The pharmacist does know which treatment the participants are given. Such blinding is designed to prevent members of the research team and study participants from influencing results.

Study Endpoints
In a clinical trial protocol, the primary endpoint is the planned outcome measure that is the most important for evaluating the effect of an intervention or treatment.
Active (Currently Enrolling) Clinical Trials
Study Title
Targeted Patients
An Open-Label, Single-Arm, Phase II, Multinational, Multicentre Study to Assess the Efficacy and Safety of 5 Years of Osimertinib in Patients with Epidermal Growth Factor Receptor Mutation Positive Stage IB-IIIA Non-Small Cell Lung Carcinoma, Following Complete Tumour Resection With or Without Adjuvant Chemotherapy (TARGET)
https://www.astrazenecaclinicaltrials.com/study/D5162C00048/
Patients with Epidermal Growth Factor Receptor Mutation Positive Stage IB-IIIA Non-Small Cell Lung Carcinoma
Feasibility Survey for Asciminib in 1L CML
A Phase IIIb, Multi-Center, Open-Label, Randomized Study of Tolerability and Efficacy of Oral Asciminib Versus Nilotinib in Patients with Newly Diagnosed Philadelphia Chromosome Positive Chronic Myelogenous Leukemia in Chronic Phase
Adult Patients with Newly Diagnosed Ph+ CML-CP
A Phase 2b, Open-Label, Multicenter, Randomized, Controlled, 2-Arm Study to Assess the Efficacy and Safety of Orally Administered NS-018 Versus Best Available Therapy in Subjects with Primary Myelofibrosis, Post-Polycythemia Vera Myelofibrosis, or Post-Essential Thrombocythemia Myelofibrosis with Severe Thrombocytopenia (Platelet Count < 50,000/μL)
Patients with Primary Myelofibrosis, Post-Polycythemia Vera Myelofibrosis, or Post-Essential Thrombocythemia Myelofibrosis with Severe Thrombocytopenia (Platelet Count < 50,000/μL)
A Randomized, Controlled, Open Label, Phase III Study Evaluating the Efficacy and Safety of JDQ443 Versus Docetaxel in Previously Treated Subjects with Locally Advanced or Metastatic KRAS G12C Mutant Non-Small Cell Lung Cancer
Individuals With Locally Advanced or Metastatic KRAS G12C Mutant Non-Small Cell Lung Cancer
Study Title
An Open-Label, Single-Arm, Phase II, Multinational, Multicentre Study to Assess the Efficacy and Safety of 5 Years of Osimertinib in Patients with Epidermal Growth Factor Receptor Mutation Positive Stage IB-IIIA Non-Small Cell Lung Carcinoma, Following Complete Tumour Resection With or Without Adjuvant Chemotherapy (TARGET)
Targeted Patients
Patients with Epidermal Growth Factor Receptor Mutation Positive Stage IB-IIIA Non-Small Cell Lung Carcinoma
Study Title
Feasibility Survey for Asciminib in 1L CML
A Phase IIIb, Multi-center, Open-label, Randomized Study of Tolerability and Efficacy of Oral Asciminib Versus Nilotinib in Patients with Newly Diagnosed Philadelphia Chromosome Positive Chronic Myelogenous Leukemia in Chronic Phase
Targeted Patients
Adult Patients with Newly Diagnosed Ph+ CML-CP
Study Title
A Phase 2b, Open-Label, Multicenter, Randomized, Controlled, 2-Arm Study to Assess the Efficacy and Safety of Orally Administered NS-018 Versus Best Available Therapy in Subjects with Primary Myelofibrosis, Post-Polycythemia Vera Myelofibrosis, or Post-Essential Thrombocythemia Myelofibrosis with Severe Thrombocytopenia (Platelet Count < 50,000/μL)
Targeted Patients
Patients with Primary Myelofibrosis, Post-Polycythemia Vera Myelofibrosis, or Post-Essential Thrombocythemia Myelofibrosis with Severe Thrombocytopenia (Platelet Count < 50,000/μL)
Study Title
A Randomized, Controlled, Open Label, Phase III Study Evaluating the Efficacy and Safety of JDQ443 Versus Docetaxel in Previously Treated Subjects with Locally Advanced or Metastatic KRAS G12C Mutant Non-Small Cell Lung Cancer
Targeted Patients
Individuals With Locally Advanced or Metastatic KRAS G12C Mutant Non-Small Cell Lung Cancer
Observational Study
An observational study is a type of research study in which researchers observe the effects of an intervention on a group of participants without intervening. Participants are not assigned to specific interventions by the investigator, as is the case in a clinical trial; however, they may be receiving a treatment that is already part of their routine medical care. Researchers can then assess the associations between interventions and health outcomes among people receiving it as part of their standard care. Such findings may lead to further investigation in a clinical trial. There are various types of observational studies, one of which is a patient registry.
A patient registry is a long-term, open-ended data collection system that uses observational methods to collect information about patients with cancer and haematologic conditions. It serves as a repository of detailed information, treatment plans and patient outcomes which can be used for ongoing research, clinical studies and informed treatment decisions.

Patient Registries

Demographic Landscape and Clinical Characteristics of Haematology Patients in a Single-Centre Private Institution in Malaysia

Demographic Landscape and Clinical Characteristics of Patients with Solid Cancers in a Single-Centre Private Institution in Malaysia

Asian Myeloproliferative Neoplasm (MPN) Registry - An Asian Myeloid Working Group (AMWG) Project

Clinical and Cenomic Registry of Myelodysplastic Syndrome and Secondary Acute Myeloid Leukaemia in Asia

The Acute Promyelocytic Leukaemia Asian Consortium Project

Asia-Pacific Myeloma and Related Diseases Registry (APAC MRDR)
Publications

Analytical and Clinical Validation of a Custom 15-Gene Next-Generation Sequencing Panel for the Evaluation of Circulating Tumor DNA Mutations in Patients with Advanced Non-Small-Cell Lung Cancer
Read More
MicroRNAs that Affect the Fanconi Anemia/BRCA Pathway Are Downregulated in Imatinib-Resistant Chronic Myeloid Leukaemia Patients Without Detectable BCR-ABL Kinase Domain Mutations
Read More
From Driver Mutations to Genomic Classification: Current & Future Perspectives on Myeloproliferative Neoplasms
Read More
Primary Imatinib Resistance in Chronic Myeloid Leukaemia Patients in a Developing Country: BCR-ABL Kinase Domain Mutations or BCR-ABL Independent Mechanisms?
Read More
Validation of a 22-Gene Next Generation Sequencing (NGS) Panel as a Molecular Diagnostic Tool for the Management of Patients with Myeloproliferative Neoplasms
Read More
Analytical and Clinical Validation of a Custom 15-Gene Next-Generation Sequencing Panel for the Evaluation of Circulating Tumor DNA Mutations in Patients with Advanced Non-Small-Cell Lung Cancer
Read More
MicroRNAs that Affect the Fanconi Anemia/BRCA Pathway Are Downregulated in Imatinib-Resistant Chronic Myeloid Leukaemia Patients Without Detectable BCR-ABL Kinase Domain Mutations
Read More
From Driver Mutations to Genomic Classification: Current & Future Perspectives on Myeloproliferative Neoplasms
Read More
Primary Imatinib Resistance in Chronic Myeloid Leukaemia Patients in a Developing Country: BCR-ABL Kinase Domain Mutations or BCR-ABL Independent Mechanisms?
Read More
Validation of a 22-Gene Next Generation Sequencing (NGS) Panel as a Molecular Diagnostic Tool for the Management of Patients with Myeloproliferative Neoplasms
Read More
Analytical and Clinical Validation of a Custom 15-Gene Next-Generation Sequencing Panel for the Evaluation of Circulating Tumor DNA Mutations in Patients with Advanced Non-Small-Cell Lung Cancer
Read More
MicroRNAs that Affect the Fanconi Anemia/BRCA Pathway Are Downregulated in Imatinib-Resistant Chronic Myeloid Leukaemia Patients Without Detectable BCR-ABL Kinase Domain Mutations
Read More
From Driver Mutations to Genomic Classification: Current & Future Perspectives on Myeloproliferative Neoplasms
Read More
Primary Imatinib Resistance in Chronic Myeloid Leukaemia Patients in a Developing Country: BCR-ABL Kinase Domain Mutations or BCR-ABL Independent Mechanisms?
Read More
